575
Views & Citations10
Likes & Shares
PREFACE
We all in the
agricultural field need to spray pesticides, fertilizers, growth regulators or
called plant hormones. We usually think about the target tissue, organ or
organelles but do not give enough attention about the first barrier of the
applied chemical. Farmers could have ample information about the dose, the
stage of plant development or even the pH of the spray solution. These above
information could be written on the stock solution package. However, we must
know that it is not enough to just spray and pray since we might not take into
consideration the actual amount that penetrated the barrier that covers the
above ground canopy which is covered by a thin or thick cuticle. The
penetration of sprayed chemicals across the plant cuticle is via the diffusion
mechanism [1].
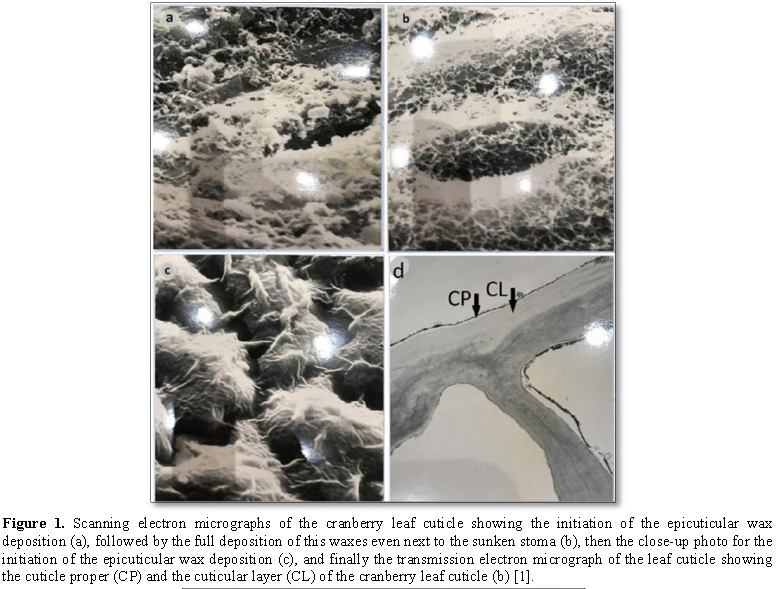
The cuticle is not
only just the waxy outermost layer that we can observe easily on the red
Delicious apple fruit. This whitish layer is only the epicuticular waxes (Figure
1). However, the cuticle is scientifically described as the cuticular
membrane that consists of many layers that have a hydrophobic nature. They are
called the cuticle proper, the cuticular layer which has many protrusions that
extend between the epidermal cells or in some plants. It extends to surround
the epidermal cells. Such cuticular layer has many embedded wax platelets that
are very resistant to penetration of sprayed materials. Fortunately, some fruit
cuticles possess few natural cracks or sometimes very tiny channels called the
micro-channels or even some lenticels representing a physical path in the
cuticle structure.
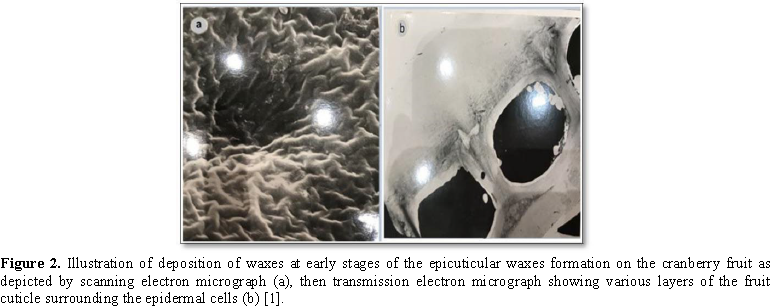
The epicuticular
wax plays very important roles (Figure 2) in reducing water loss,
controlling gaseous exchange, restricting losses of nutrients and retaining
traces of foliar applied chemicals. It is also a good micro-habitat for fungal
pathogens. Such thin waxy layer can also reflect some incident radiations. The
rate of movement and transport across that cuticular construction depends on
many factors since it is a diffusion mechanism. These factors are governing the
actual penetration process reported by Fick's Low [2] such as temperature,
partition coefficient of chemicals in the sprayed droplets, radius and
tortuosity of the cuticular-layer micro-spaces and the concentration gradient
between sprayed solution and the internal solution in the extracellular spaces
between cells. Thus, it seems a difficult task to diffuse the desired compound
through the cuticle. The embedded waxes or also called the intra-cuticular
waxes are very tough to penetrate. It is not surprising to find a thin cuticle
as in ripe tomato fruit more resistant to diffusion of sprayed chemicals than
that of the thicker apple fruit cuticle [3-10].
This information
must be considered when taking the decision to spray at a certain stage of
fruit development in order to get the expected results. Many farmers rush into
applying a second and a third spray to see the outcome they are after such as
early coloration or ripening or acceleration of the harvest time before the
frost season. Chemicals that release ethylene inside the tissue such as the
plant growth regulator called ethephon (or Ethrel) have a very hydrophilic
nature with low ability to penetrate the hydrophobic-cuticular layers. The
repeated application to increase its efficacy might lead to more abscission and
shorten the storage life of fruit whether during the cold storage or at ambient
temperature on the shelf. The use of surfactants along with sprayed chemicals
is thought about some growers. However, these surfactants do not increase the
actual diffusion but rather increase the contact angle of sprayed droplets and
reduce the surface tension of sprayed solution drops. In some cases, it was
helpful to prolong the drying time of sprayed solutions by adding small amount
of glycerol [1]. It was also beneficial to incorporate some ethanol in the sprayed formulation
since it was found that
Fore mentioned information emphasizes the
significance of applying sprayed chemicals in formulations that enhance the
diffusion and reduce the cost and reduce environmental pollution. No wonder, it
has been very important to widely use a natural compound called
Lysophosphatidyl Ethanolamine (LPE) as a plant and fruit growth regulator that
has been granted the approval of Food and drug Administration in the USA and
has been applied in countries such as the USA, Spain,
Portugal, Turkey, South Africa, South Korea and Egypt. The USA Patent Office
granted four patents to the first author of this article [6]. Such natural
compound is a lysophospholipid with a hydrophobic nature and mitigates the
adverse effects of Ethrel. At a certain concentration, LPE stimulates ethylene
production without an accompanied rise of respiration rate [5]. Moreover, LPE
was able to avoid the adverse effects of ethephon on enhancing ripening of
tomato without damaging the leaves [8]. It also alleviated stresses of some
pesticides or environmental ones. In following studies, Ryu et al. [9], showed
that LPE is the first inhibitor of the senescence enzyme called phospholipase
D. In addition, LPE caused novel effects on plants and fruits such as retarding
and delaying tissue senescence while enhancing fruit coloration [10,11-17] and
extending their keeping quality [8,13,14] and extended the vase life of cut
flowers [15].
1. Farag KM (1989) Enhancing ethephon
effectiveness by modifying cuticular transport or stimulating ethylene
production in cranberry fruit. Ph.D. Thesis. University of Wisconsin, Madison,
p: 216.
2. Fick A (1855) On liquid diffusion.
J Membr Sci 100: 33-38.
3. Farag KM, Palta JP, Stage EJ
(1992) Ethanol enhances the effectiveness of ethephon on anthocyanin production
in cranberry fruits in the field. Hort Sci 27: 411-412.
4. Farag KM, Palta JP, Stang EJ
(1989) Field application of new ethrel formulations for early color enhancement
in cranberry (Vaccinium macrocarpon
Ait). Fourth International Symposium of Vaccinium culture. Acta Hort 241:
373-375.
5. Farag KM, Palta JP (1989)
Stimulation of ethylene production by urea, thidiazuron
lysophosphatidyl-ethanolamine and possible sites of stimulation. Plant Physiol
89: 568.
6. Farag KM, Palta JP, Steven RB
(2003) Methods for enhancing plant health, protecting plants from biotic and
abiotic stress related injuries and enhancing the recovery of plants injured as
a result of such stresses. Granted USA Patent number 6,559, 099 BI. Granted on
May 6, 2003.
7. Farag KM, Palta JP (1989)
Ultrastructure and surface morphology of cranberry plant (Vaccinium macrocarpon Ait) with reference to ethrel penetration.
Fourth International Symposium of Vaccinium culture. Acta Hort 241: 378-384.
8. Farag KM, Palta JP (1993) Use of
natural lipids to accelerate ripening and enhance storage life of tomato fruit
with without ethephon. Hort Technol 3: 62-65.
9. Ryu S, Karlsson BH, Ozgen M, Palta
JP (1997) Inhibition of phospholipase D by lysophosphatidyl-ethanolamine, a
lipid-derived senescence retardant. Proc Natl Acad Sci U S A 94: 12717-12721.
10. Ozgen, M, Farag KM, Ozgen S, Palta
JP (2004) Lysophosphatidyl ethanolamine accelerates color development and
promotes shelf life of cranberries. Hort Sci 40: 127-130.
11. Farag KM, Palta JP (1990) Use of
lysophosphatidyl-ethanolamine, a natural lipid, as aid for fruit ripening and
improving keeping quality. Proceedings of 17th Annual Meeting of
Plant Growth Regulators Society of America, pp: 135-137.
12. Farag KM, Palta JP (1991) Use of
lysophosphatidyl-ethanolamine, a natural lipid, to delay tomato fruit and leaf
senescence. Hort Sci 26: 11.
13. Farag KM, Palta JP (1993) Use of
lysophosphatidyl-ethanolamine, a natural lipid, to retard tomato leaf and fruit
senescence. Physiol Plant 87: 515-524.
14. Cowan K (2009) Plant growth
promotion by 18:0-Lysophosphatidylethanolamine involves senescence delay. Plant
Signal Behav 4: 324-327.
15. Kaur N, Palta JP (1996)
Post-harvest dipping in natural lipid, lysophosphotidyl ethanolamine, may
prolong vase life of Snapdragon flowers. Hort Sci 3265: 888-890.
16. Farag KM, Palta JP (1991)
Improving postharvest keeping quality of vine-ripened tomato fruits with a
natural lipid. Hort Sci 26: 126.
17. Farag KM, Palta JP (1991)
Enhancing ripening and keeping quality of apple and cranberry fruits using
lysophosphatidyl ethanolamine, a natural lipid. Hort Sci 26: 67.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- Proteomics and Bioinformatics (ISSN:2641-7561)
- Food and Nutrition-Current Research (ISSN:2638-1095)
- Journal of Veterinary and Marine Sciences (ISSN: 2689-7830)
- Journal of Genetics and Cell Biology (ISSN:2639-3360)
- Journal of Biochemistry and Molecular Medicine (ISSN:2641-6948)
- Journal of Genomic Medicine and Pharmacogenomics (ISSN:2474-4670)
- Journal of Astronomy and Space Research